Aging is a complex and multifaceted process that affects various aspects of an organism's biology, including its immune system. As organisms age, their ability to defend against pathogens and infections often declines, making them more susceptible to disease. This decline in immune function is particularly significant in the context of antiviral defense mechanisms, which are crucial for protecting against viral infections.
Caenorhabditis elegans, a nematode worm, is a popular model organism used to study the effects of aging on immune function. Research on C. elegans has shown that aging has a profound impact on its antiviral defense mechanisms. This introduction aims to explain the impact of aging on antiviral defense mechanisms in C. elegans, with a focus on the key factors involved.
Some of the key aspects of antiviral defense mechanisms in C. elegans that are affected by aging include:
- Decline in immune cell function
- Changes in gene expression
- Alterations in signaling pathways
- Increased susceptibility to viral infections
These changes can have significant consequences for the health and survival of C. elegans, and may also have implications for our understanding of aging and immune function in other organisms.
The study of antiviral defense mechanisms in C. elegans has the potential to provide valuable insights into the effects of aging on immune function, and may also inform the development of new strategies for promoting healthy aging and preventing age-related diseases. By exploring the complex relationships between aging, immune function, and antiviral defense mechanisms in C. elegans, researchers can gain a deeper understanding of the underlying biological processes and identify potential targets for intervention.

Introduction to Caenorhabditis elegans
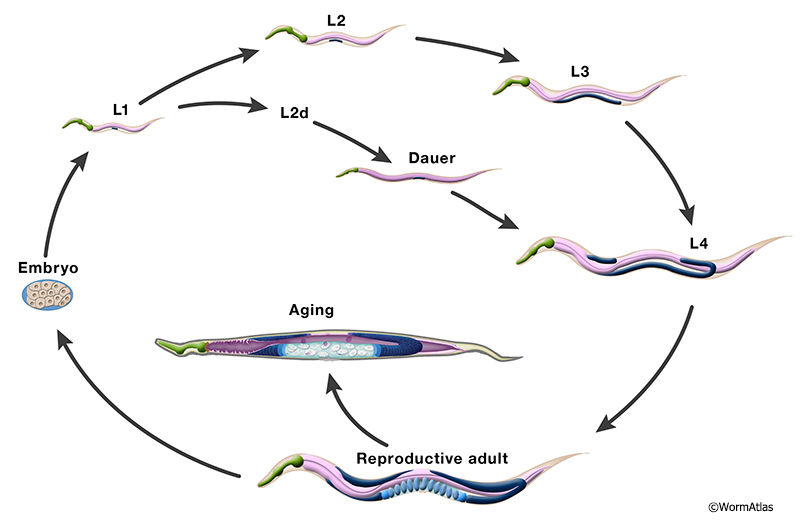
Caenorhabditis elegans, commonly referred to as C. elegans, is a free-living, transparent nematode worm that is about 1 millimeter in length. It is a widely used model organism in scientific research due to its well-characterized genome, short life cycle, and ease of cultivation in the laboratory.
One of the key reasons C. elegans is used in scientific research is its genetic similarity to humans. Despite being a simple worm, it has a significant number of genes that are similar to those found in humans, making it an ideal model for studying various biological processes. This genetic similarity, combined with its simplicity and ease of manipulation, has made C. elegans a popular choice for researchers studying a wide range of topics, from development and neurobiology to disease and behavior.
The use of C. elegans in studying aging is a significant area of research. Some of the key areas of focus include:
- Understanding the genetic and molecular mechanisms that regulate aging and longevity
- Identifying the role of specific genes and pathways in the aging process
- Developing interventions that can promote healthy aging and increase lifespan
By studying aging in C. elegans, researchers can gain valuable insights into the underlying mechanisms of aging and develop new strategies for promoting healthy aging in humans.
In addition to its use in studying aging, C. elegans is also used to study antiviral defense. C. elegans has a relatively simple immune system, but it is still capable of mounting an effective defense against viral infections. Researchers use C. elegans to study the mechanisms of antiviral defense, including:
- The role of specific genes and pathways in recognizing and responding to viral infections
- The mechanisms of immune evasion used by viruses to avoid detection by the host immune system
- The development of new antiviral therapies that can be used to treat viral infections in humans
Overall, C. elegans is a powerful model organism that has contributed significantly to our understanding of a wide range of biological processes, including aging and antiviral defense. Its use in scientific research continues to grow, and it is likely to remain a key model organism for many years to come.
The Role of DRH-1/RIG-I in Antiviral Defense
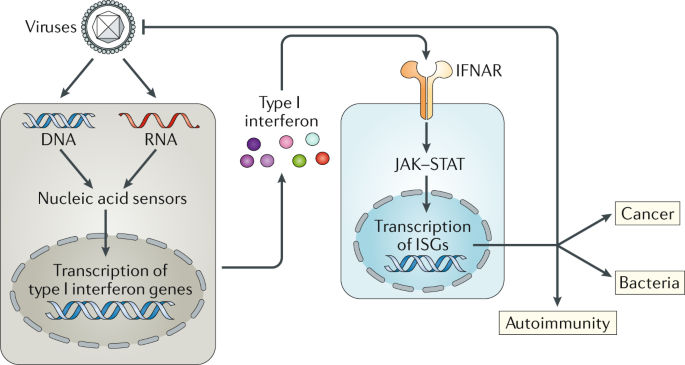
The recognition of viral RNA by pattern recognition receptors (PRRs) is a crucial step in the activation of antiviral defense mechanisms. DRH-1/RIG-I is a key player in this process, functioning as a cytoplasmic sensor that recognizes viral RNA and triggers a signaling cascade to initiate an antiviral response.
DRH-1/RIG-I recognizes viral RNA through its helicase domain, which binds to specific RNA structures and motifs. This recognition event leads to a conformational change in the protein, activating its signaling potential. The activated DRH-1/RIG-I then interacts with downstream signaling molecules to trigger an antiviral response.
The antiviral response triggered by DRH-1/RIG-I involves the activation of various signaling pathways, including the production of type I interferons (IFNs) and the activation of inflammatory responses. The key steps involved in this process are:
- Recognition of viral RNA by DRH-1/RIG-I
- Activation of the signaling cascade, leading to the production of IFNs
- Induction of IFN-stimulated genes (ISGs), which have antiviral properties
- Activation of inflammatory responses, which help to eliminate infected cells
The importance of DRH-1/RIG-I deSUMOylation in antiviral defense cannot be overstated. DeSUMOylation is a post-translational modification that involves the removal of small ubiquitin-like modifier (SUMO) proteins from target proteins. In the case of DRH-1/RIG-I, deSUMOylation is required for its activation and signaling potential. The deSUMOylation of DRH-1/RIG-I allows it to interact with downstream signaling molecules and trigger an antiviral response.
The regulation of DRH-1/RIG-I activity by deSUMOylation is a complex process that involves the coordinated action of multiple enzymes and proteins. The deSUMOylation of DRH-1/RIG-I is mediated by specific enzymes, which remove SUMO proteins from the protein and activate its signaling potential. This process is critical for the initiation of an antiviral response and the elimination of viral infections.
Impact of Aging on DRH-1/RIG-I deSUMOylation
Aging is a complex and multifaceted process that affects various cellular mechanisms, including the regulation of immune responses. One such mechanism is the deSUMOylation process of DRH-1/RIG-I, which plays a crucial role in antiviral defense. The deSUMOylation process involves the removal of small ubiquitin-like modifier (SUMO) proteins from target proteins, including DRH-1/RIG-I, to regulate their activity and localization.
The role of ULP-4/SENP7 in the deSUMOylation process of DRH-1/RIG-I is significant. ULP-4/SENP7 is a SUMO-specific protease that catalyzes the removal of SUMO proteins from DRH-1/RIG-I, thereby regulating its activity and antiviral functions. The activity of ULP-4/SENP7 is essential for maintaining the proper regulation of DRH-1/RIG-I and ensuring effective antiviral defense.
As individuals age, the deSUMOylation process of DRH-1/RIG-I can become impaired, leading to consequences for antiviral defense. Some of the key consequences include:
- Impaired recognition of viral RNA by DRH-1/RIG-I, leading to reduced activation of downstream signaling pathways
- Decreased production of type I interferons and other pro-inflammatory cytokines, which are essential for antiviral defense
- Increased susceptibility to viral infections, which can lead to severe disease and poor outcomes in older adults
The impairment of deSUMOylation can be due to various factors, including decreased expression or activity of ULP-4/SENP7, as well as increased expression of SUMO proteins that can inhibit the activity of ULP-4/SENP7.
The consequences of impaired deSUMOylation on antiviral defense can be severe, particularly in older adults who are already more susceptible to viral infections. The impairment of DRH-1/RIG-I function can lead to a range of negative outcomes, including increased morbidity and mortality from viral infections. Therefore, understanding the mechanisms that regulate the deSUMOylation process of DRH-1/RIG-I and how they are affected by aging is essential for developing effective therapeutic strategies to enhance antiviral defense in older adults.

Consequences of Impaired Antiviral Defense in Aging
As people age, their immune system undergoes significant changes, leading to impaired antiviral defense. This impairment makes older adults more susceptible to viral infections, which can have severe consequences on their overall health and longevity. The increased susceptibility to viral infections with age can be attributed to the decline in the function of immune cells, such as T cells and B cells, which play a crucial role in fighting off viral infections.
The decline in antiviral defense with age can lead to a range of health problems, including increased severity and duration of viral infections, such as influenza and herpes zoster. Additionally, older adults may be more likely to experience complications from viral infections, such as pneumonia and bronchitis, which can be life-threatening. The potential consequences of impaired antiviral defense on overall health and longevity include:
- Increased risk of hospitalization and mortality from viral infections
- Decreased quality of life due to chronic illness and disability
- Increased risk of age-related diseases, such as dementia and cardiovascular disease
The potential avenues for further research into the consequences of impaired antiviral defense in aging include investigating the underlying mechanisms of immune decline with age, as well as exploring new strategies for boosting antiviral immunity in older adults. Some potential areas of research include:
- Developing vaccines that are specifically designed for older adults
- Investigating the role of nutrition and lifestyle in maintaining antiviral immunity with age
- Exploring the potential of immunotherapies, such as checkpoint inhibitors, to enhance antiviral immunity in older adults
Further research into the consequences of impaired antiviral defense in aging is crucial to developing effective strategies for preventing and treating viral infections in older adults. By understanding the underlying mechanisms of immune decline with age, researchers can identify potential targets for intervention and develop new therapies to enhance antiviral immunity and promote healthy aging. This research has the potential to improve the health and well-being of older adults, reducing the burden of viral infections and promoting a longer and healthier life.
Frequently Asked Questions (FAQ)
What is DRH-1/RIG-I and its role in antiviral defense?
The recognition of viral RNA by the host cell is a critical step in the initiation of an antiviral response. DRH-1/RIG-I is a protein that plays a central role in this process. It is a cytoplasmic sensor that recognizes viral RNA and triggers a signaling cascade that ultimately leads to the production of antiviral cytokines.
This protein is able to recognize viral RNA through its helicase domain, which binds to the RNA and activates the protein. Once activated, DRH-1/RIG-I interacts with downstream signaling molecules, including the adaptor protein MAVS, to initiate the antiviral response. The activation of this pathway leads to the production of type I interferons, which are critical for protecting against viral infections.
Some of the key features of DRH-1/RIG-I include:
- Recognition of viral RNA: DRH-1/RIG-I is able to recognize a wide range of viral RNAs, including those from influenza, HIV, and hepatitis C
- Activation of antiviral signaling: DRH-1/RIG-I triggers a signaling cascade that leads to the production of antiviral cytokines
- Interaction with downstream signaling molecules: DRH-1/RIG-I interacts with MAVS and other signaling molecules to initiate the antiviral response
The role of DRH-1/RIG-I in antiviral defense is crucial for protecting against viral infections. By recognizing viral RNA and triggering an antiviral response, this protein helps to limit the spread of viral infections and prevent disease. In addition, DRH-1/RIG-I has been implicated in the regulation of immune responses, including the activation of immune cells and the production of cytokines.
In summary, DRH-1/RIG-I is a critical component of the antiviral response, recognizing viral RNA and triggering a signaling cascade that leads to the production of antiviral cytokines. Its role in protecting against viral infections makes it an important area of study for the development of new antiviral therapies.
How does aging affect the antiviral defense mechanism in Caenorhabditis elegans?
Aging is a complex and multifaceted process that affects various biological systems, including the immune system. In Caenorhabditis elegans, a widely used model organism, aging has been shown to impair the antiviral defense mechanism. This impairment makes the organism more susceptible to viral infections, which can have severe consequences for its survival and overall health.
The antiviral defense mechanism in Caenorhabditis elegans involves a complex interplay of various molecular pathways. One of the key components of this mechanism is the deSUMOylation of DRH-1/RIG-I, a protein that plays a crucial role in recognizing and responding to viral infections. DeSUMOylation is a post-translational modification process that involves the removal of small ubiquitin-like modifier (SUMO) proteins from target proteins.
The deSUMOylation of DRH-1/RIG-I is essential for its activation and ability to respond to viral infections. However, as Caenorhabditis elegans ages, the deSUMOylation process is impaired, leading to a decrease in the activation of DRH-1/RIG-I. This impairment has significant consequences for the antiviral defense mechanism, making the organism more vulnerable to viral infections. Some of the key consequences of impaired deSUMOylation of DRH-1/RIG-I include:
- Decreased ability to recognize and respond to viral infections
- Impaired activation of downstream signaling pathways
- Reduced production of antiviral genes and proteins
- Increased susceptibility to viral infections and disease
The impairment of the antiviral defense mechanism in aging Caenorhabditis elegans is a complex process that involves multiple molecular pathways and mechanisms. Further research is needed to fully understand the underlying mechanisms and to identify potential therapeutic targets for improving antiviral defense in aging organisms. By studying the effects of aging on the antiviral defense mechanism in Caenorhabditis elegans, researchers can gain valuable insights into the underlying biology of aging and immunity, and develop new strategies for promoting healthy aging and preventing age-related diseases.
What are the potential consequences of impaired antiviral defense in aging?
As people age, their immune system undergoes natural changes that can affect its ability to fight off infections. One critical aspect of the immune system is antiviral defense, which plays a vital role in protecting the body against viral infections. Impaired antiviral defense in aging can have significant consequences, making older adults more vulnerable to viral infections.
The potential consequences of impaired antiviral defense in aging include increased susceptibility to viral infections, which can lead to a range of health problems and potentially affect overall longevity. This is because older adults may have a reduced ability to mount an effective immune response against viruses, making them more likely to experience severe illness and complications.
Some of the potential health problems associated with impaired antiviral defense in aging include:
- Respiratory infections, such as pneumonia and influenza
- Gastrointestinal infections, such as norovirus and rotavirus
- Neurological infections, such as herpes simplex and West Nile virus
- Increased risk of secondary bacterial infections, which can be life-threatening
These infections can lead to significant morbidity and mortality in older adults, highlighting the importance of maintaining effective antiviral defense as we age.
In addition to the immediate health problems caused by viral infections, impaired antiviral defense in aging can also have long-term consequences. For example, repeated or chronic viral infections can lead to inflammation and tissue damage, which can contribute to the development of chronic diseases such as cardiovascular disease and dementia. Furthermore, impaired antiviral defense can also affect the overall quality of life and independence of older adults, making it essential to understand the mechanisms underlying impaired antiviral defense in aging and to develop effective strategies to prevent or mitigate its consequences.




