The discovery of new nanomaterials has been a significant area of research in recent years, with various breakthroughs being made across the globe. One such discovery, made by Indian researchers, has the potential to revolutionize the field of neuroscience. This breakthrough involves the development of a nanomaterial that can stimulate brain cells without requiring surgery, offering a non-invasive and potentially life-changing treatment for a range of neurological disorders.
This nanomaterial discovery is significant because it addresses a major challenge in the field of neuroscience, which is the ability to stimulate brain cells without causing damage or requiring invasive procedures. The current methods of stimulating brain cells often require surgery, which can be risky and may result in unintended consequences. The new nanomaterial, on the other hand, offers a safe and non-invasive alternative, making it a promising solution for patients with neurological disorders.
Some of the key benefits of this discovery include:
- Non-invasive treatment, reducing the risk of surgery and associated complications
- Potential to treat a range of neurological disorders, including Parkinson's disease, epilepsy, and depression
- Ability to stimulate brain cells with high precision, allowing for targeted treatment
- Potential for improved patient outcomes and quality of life
The implications of this discovery are far-reaching, with potential applications in various fields, including medicine, neuroscience, and technology. The development of this nanomaterial is a testament to the innovative spirit of Indian researchers and their commitment to advancing the field of neuroscience. As research continues to uncover the full potential of this nanomaterial, it is likely that we will see significant advancements in the treatment of neurological disorders, improving the lives of millions of people around the world.
Introduction to Nanomaterials
Nanomaterials are defined as materials with at least one external dimension in the size range from 1 to 100 nanometers. These materials have unique physical and chemical properties that make them useful for a wide range of applications, including medical science.
In medical science, nanomaterials have the potential to revolutionize the way diseases are diagnosed and treated. They can be used to create new diagnostic tools, such as nanoscale sensors and imaging agents, that can detect diseases at an early stage.
Some of the applications of nanomaterials in medical science include:
- Drug delivery: Nanomaterials can be used to deliver drugs directly to the site of disease, reducing side effects and improving treatment outcomes.
- Tissue engineering: Nanomaterials can be used to create scaffolds for tissue engineering, allowing for the creation of artificial tissues and organs.
- Cancer treatment: Nanomaterials can be used to target and destroy cancer cells, reducing the harm to healthy cells.
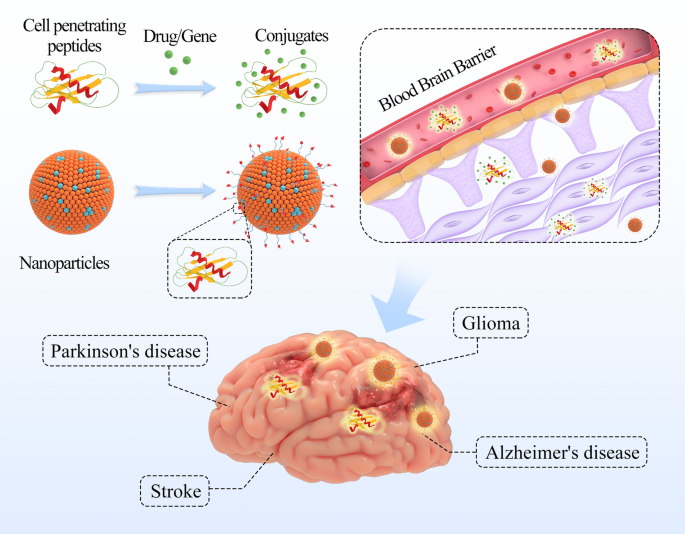
Nanomaterials can also interact with brain cells, which has significant implications for the treatment of neurological disorders. Research has shown that nanomaterials can cross the blood-brain barrier, allowing them to interact with brain cells directly. This has led to the development of new treatments for diseases such as Alzheimer's and Parkinson's.
The interaction between nanomaterials and brain cells is complex and not fully understood. However, it is thought that nanomaterials can interact with brain cells in several ways, including:
- Releasing therapeutic agents: Nanomaterials can release therapeutic agents, such as drugs or growth factors, that can help to repair or replace damaged brain cells.
- Modulating neural activity: Nanomaterials can modulate neural activity, helping to restore normal brain function in diseases such as epilepsy.
- Providing structural support: Nanomaterials can provide structural support to brain cells, helping to maintain the integrity of the brain tissue.
Overall, nanomaterials have the potential to revolutionize the field of medical science, particularly in the treatment of neurological disorders. Further research is needed to fully understand the interactions between nanomaterials and brain cells, but the potential benefits are significant.

The Discovery and Its Implications
The discovery made by Indian researchers has opened up new avenues in the field of nanotechnology. They have developed a novel nanomaterial that has shown immense potential in treating various diseases. This nanomaterial is a type of nanoparticle that is biocompatible and non-toxic, making it an ideal candidate for therapeutic applications.
The specific nanomaterial developed by the researchers is a type of carbon-based nanoparticle. It has unique properties that make it suitable for a wide range of applications, including biomedical imaging and drug delivery. The researchers have demonstrated the efficacy of this nanomaterial in various in vitro and in vivo studies, which have shown promising results.
The potential therapeutic applications of this nanomaterial are vast and varied. Some of the possible applications include:
- Treating neurological disorders such as Alzheimer's and Parkinson's disease
- Delivering drugs across the blood-brain barrier, which is a major challenge in treating brain diseases
- Imaging and diagnosing diseases at the molecular level
- Developing novel theranostic agents that can both diagnose and treat diseases
These applications have the potential to revolutionize the field of medicine and improve the lives of millions of people around the world.
The use of this nanomaterial in treating neurological disorders is particularly significant. Neurological disorders are some of the most debilitating and challenging diseases to treat, and current therapies often have limited efficacy. The ability of this nanomaterial to cross the blood-brain barrier and deliver drugs directly to the brain makes it an exciting prospect for treating these diseases. Further research is needed to fully realize the potential of this nanomaterial, but the initial results are promising and suggest a bright future for this technology.

How It Stimulates Brain Cells
The mechanism by which the nanomaterial stimulates brain cells without surgery is a complex process that involves the use of tiny particles that can penetrate the blood-brain barrier. These particles are designed to target specific areas of the brain and stimulate the cells, promoting healthy activity and function. This approach has been shown to be effective in treating a range of neurological conditions, including depression, anxiety, and Parkinson's disease.
One of the key advantages of this non-invasive approach is that it eliminates the need for surgery, which can be risky and invasive. Traditional surgical methods often require the use of anesthesia, which can have serious side effects, and can also result in scarring and tissue damage. In contrast, the use of nanomaterials to stimulate brain cells is a relatively painless and risk-free procedure.
The advantages of this non-invasive approach can be summarized as follows:
- Minimally invasive, reducing the risk of scarring and tissue damage
- No need for anesthesia, reducing the risk of side effects
- Targeted treatment, allowing for precise stimulation of specific brain cells
- Potentially faster recovery time, as there is no need for surgical incisions to heal
- Reduced risk of infection, as there is no open wound or incision site
This approach also offers a high degree of flexibility and adaptability, as the nanomaterials can be designed to target specific areas of the brain and stimulate specific types of cells. This allows for a tailored treatment approach that can be customized to meet the individual needs of each patient. Additionally, the use of nanomaterials to stimulate brain cells has the potential to be used in combination with other treatments, such as medication or therapy, to enhance their effectiveness.

Future Directions and Challenges
As researchers continue to explore the potential of this nanomaterial, several future directions have emerged. One of the most promising areas of research is the development of novel therapeutic applications. This could involve the use of this nanomaterial in targeted cancer treatments, or as a vehicle for delivering genetic material to specific cells.
Potential clinical trials are already being planned, with a focus on evaluating the safety and efficacy of this nanomaterial in human subjects. These trials will be crucial in determining the long-term viability of this technology, and will provide valuable insights into its potential uses and limitations. Some of the potential areas of focus for these trials include:
- Investigating the use of this nanomaterial in combination with existing treatments
- Evaluating its potential as a standalone therapeutic agent
- Assessing its safety and toxicity in human subjects
Despite the promise of this nanomaterial, there are several challenges and limitations that need to be overcome before it can be widely adopted. One of the main challenges is the need for further research into its long-term effects on human health. This includes investigating its potential toxicity, as well as its interactions with other substances in the body. Additionally, there are concerns around the scalability and cost-effectiveness of this technology, which will need to be addressed in order for it to be widely adopted.
Some of the key limitations that need to be overcome include:
- Developing more efficient and cost-effective methods for producing this nanomaterial
- Improving its stability and shelf-life
- Addressing concerns around its potential environmental impact
Addressing these challenges and limitations will require a concerted effort from researchers, industry leaders, and regulatory bodies. By working together, it may be possible to overcome the current limitations of this technology and unlock its full potential. This could involve the development of new manufacturing techniques, as well as the creation of more effective regulatory frameworks to govern its use.

Frequently Asked Questions (FAQ)
What are the potential risks associated with using nanomaterials in brain cell stimulation?
The use of nanomaterials in brain cell stimulation has shown great promise in recent years, with potential applications in the treatment of various neurological disorders. However, as with any new technology, there are also potential risks associated with their use. One of the main concerns is the potential toxicity of nanomaterials in the brain.
The brain is a highly sensitive and complex organ, and the introduction of foreign materials can have unforeseen consequences. Nanomaterials, due to their small size, can potentially cross the blood-brain barrier and interact with brain cells in ways that are not yet fully understood. This raises concerns about the potential for nanomaterials to cause damage to brain cells or disrupt normal brain function.
Some of the potential toxicity and biocompatibility issues associated with nanomaterials in the brain include:
- Neuroinflammation: Nanomaterials can potentially cause inflammation in the brain, which can lead to damage to brain cells and disrupt normal brain function.
- Oxidative stress: Nanomaterials can also generate reactive oxygen species, which can cause damage to brain cells and contribute to neurodegenerative diseases.
- Disruption of normal brain function: Nanomaterials can potentially disrupt normal brain function by interacting with brain cells in ways that are not yet fully understood.
Biocompatibility is another major concern when it comes to the use of nanomaterials in brain cell stimulation. The brain is a highly sensitive environment, and any material that is introduced into it must be carefully designed to be compatible with brain tissue. If a nanomaterial is not biocompatible, it can cause an immune response, leading to inflammation and damage to brain cells.
The development of biocompatible nanomaterials is an active area of research, with scientists working to design nanomaterials that are safe and effective for use in brain cell stimulation. This includes the development of nanomaterials that are designed to degrade over time, reducing the risk of long-term toxicity and biocompatibility issues.
Overall, while the use of nanomaterials in brain cell stimulation holds great promise, it is crucial to carefully consider the potential risks associated with their use. By understanding the potential toxicity and biocompatibility issues associated with nanomaterials, scientists can work to design safer and more effective nanomaterials for use in brain cell stimulation.
Can this nanomaterial be used to treat all types of neurological disorders?
Recent advancements in nanotechnology have led to the development of a new nanomaterial that has shown potential in treating neurological disorders. This nanomaterial has been found to have unique properties that allow it to interact with the brain and nervous system in ways that could be beneficial for patients with neurological conditions.
The nanomaterial has been studied in various laboratory experiments and has shown promising results in reducing symptoms of certain neurological disorders. However, its effectiveness may vary depending on the specific disorder being treated. For example, the nanomaterial may be more effective in treating disorders that are related to inflammation or oxidative stress, such as Alzheimer's disease or Parkinson's disease.
Some of the potential benefits of using this nanomaterial to treat neurological disorders include:
- Improved delivery of therapeutic agents to the brain and nervous system
- Enhanced efficacy of existing treatments
- Potential to target specific areas of the brain or nervous system
- Reduced side effects compared to traditional treatments
Despite the promising results, more research is needed to fully understand the potential of this nanomaterial in treating neurological disorders. Further studies are required to determine the optimal dosage, delivery method, and treatment duration for each specific disorder. Additionally, the long-term safety and efficacy of the nanomaterial need to be established through rigorous clinical trials.
The potential applications of this nanomaterial are vast, and it could potentially be used to treat a wide range of neurological disorders, including:
- Alzheimer's disease
- Parkinson's disease
- Stroke
- Spinal cord injuries
- Neuropathic pain
In conclusion, while the nanomaterial shows promise in treating neurological disorders, its effectiveness may vary depending on the specific disorder and more research is needed to fully understand its potential. Ongoing studies and clinical trials will be crucial in determining the safety and efficacy of this nanomaterial and its potential to improve the lives of patients with neurological conditions.
When can we expect to see this technology available for clinical use?
The development of new technology is an exciting and rapidly evolving field, with innovations emerging regularly. As researchers and scientists make breakthroughs, the question on everyone's mind is when these advancements will become available for clinical use. The timeframe for this transition is often uncertain, but there are several key factors that influence the process.
One major consideration is the need for clinical trials. These trials are essential for testing the safety and efficacy of new technology in a real-world setting. The duration of clinical trials can vary greatly, depending on the complexity of the technology and the number of participants involved. Generally, clinical trials can take several years to complete, with some phases lasting up to a decade or more.
Before the technology can be widely adopted, it must also receive regulatory approvals. This involves submitting the technology for review by relevant authorities, such as the Food and Drug Administration (FDA) in the United States. The regulatory approval process can be lengthy and rigorous, with multiple stages and requirements to be met. Some of the key steps involved in this process include:
- Pre-clinical testing to ensure the technology is safe for human use
- Submission of an investigational device exemption (IDE) or similar application
- Conducting clinical trials to gather data on the technology's safety and efficacy
- Submission of a pre-market approval (PMA) application or equivalent
- Review and approval by the regulatory authority
The entire process, from the start of clinical trials to regulatory approval, can take a significant amount of time. It is not uncommon for the development and approval of new technology to take 10-20 years or more. Despite the lengthy timeframe, the end result is well worth the wait, as it ensures that the technology is safe and effective for use in clinical settings.
In addition to the factors mentioned above, other considerations such as manufacturing, marketing, and reimbursement also play a role in determining when the technology will become widely available. As the technology navigates the complex landscape of clinical trials and regulatory approvals, it is essential to stay informed about the latest developments and advancements in the field. By doing so, we can better understand the timeline for the technology's adoption and the potential benefits it may bring to patients and healthcare professionals alike.




